
Electron Configuration Of Oxygen In Ground State
Wayne Breslyn 728K subscribers Join Subscribe Subscribed 799 130K views 4 years ago In this video we will write the electron configuration for O 2-, the Oxide ion. We'll also look at why Oxygen.
What is the Electron Configuration of Oxygen Archives Dynamic
0:00 / 1:20 Oxygen Electron Configuration Wayne Breslyn 724K subscribers Join Subscribe Subscribed 683 Share 130K views 10 years ago A step-by-step description of how to write the electron.
What Is the Oxygen Electron Configuration(O)?
Let's find the electron configuration of Oxygen! A single oxygen atom has 8 protons and 8 electrons, but how do we know where Oxygen puts its electrons, in w.
Electronic Configuration For Oxygen spdf Trick Chemistry Atomic
The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 8.3. 3 or 8.3. 4 ). Thus, the electron configuration and orbital diagram of lithium are:

【5 Steps】Oxygen Electron Configuration in Just 5 Steps Electron
Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. As we know, the positively-charged protons in the nucleus of an atom tend to attract negatively-charged electrons.
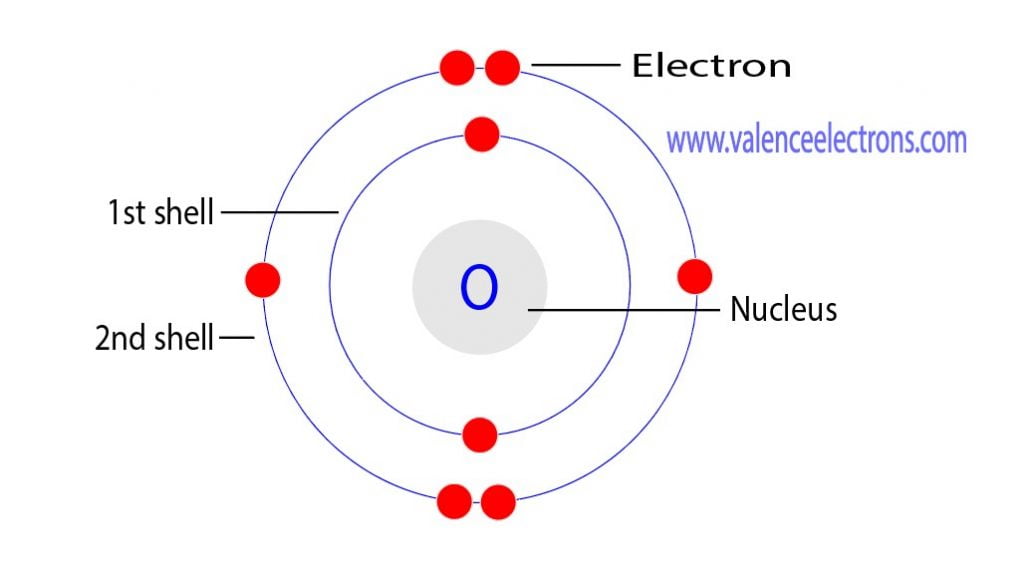
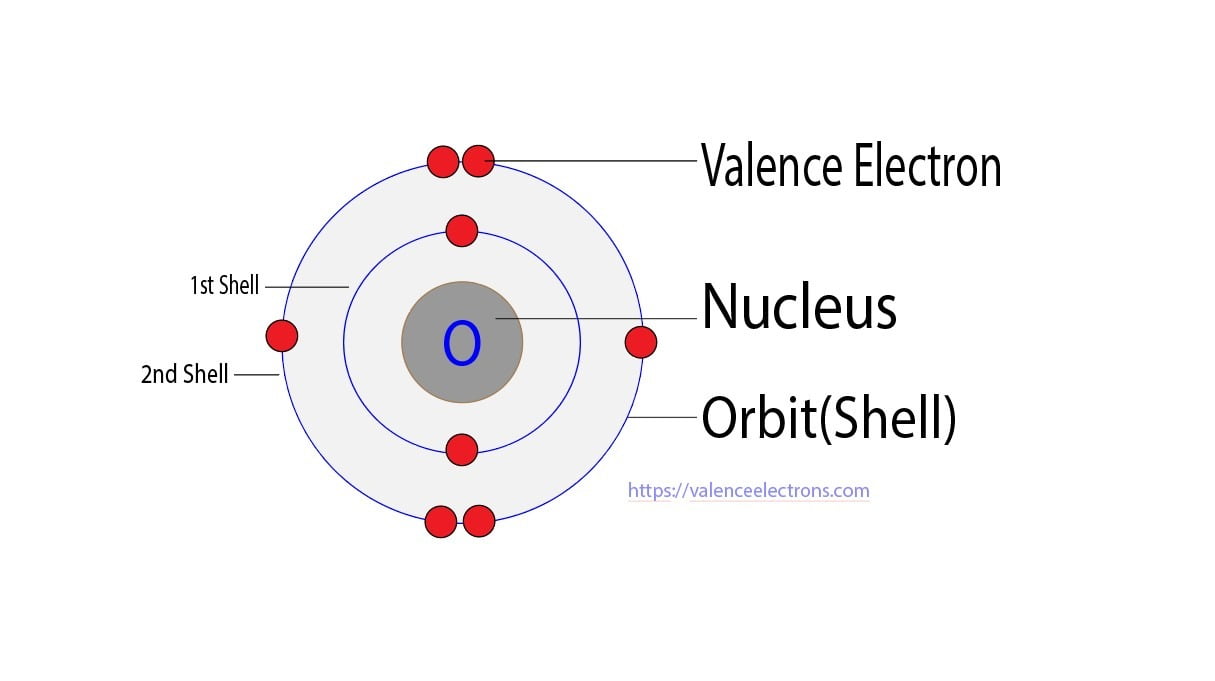
How to Find the Valence Electrons for Oxygen (O)?
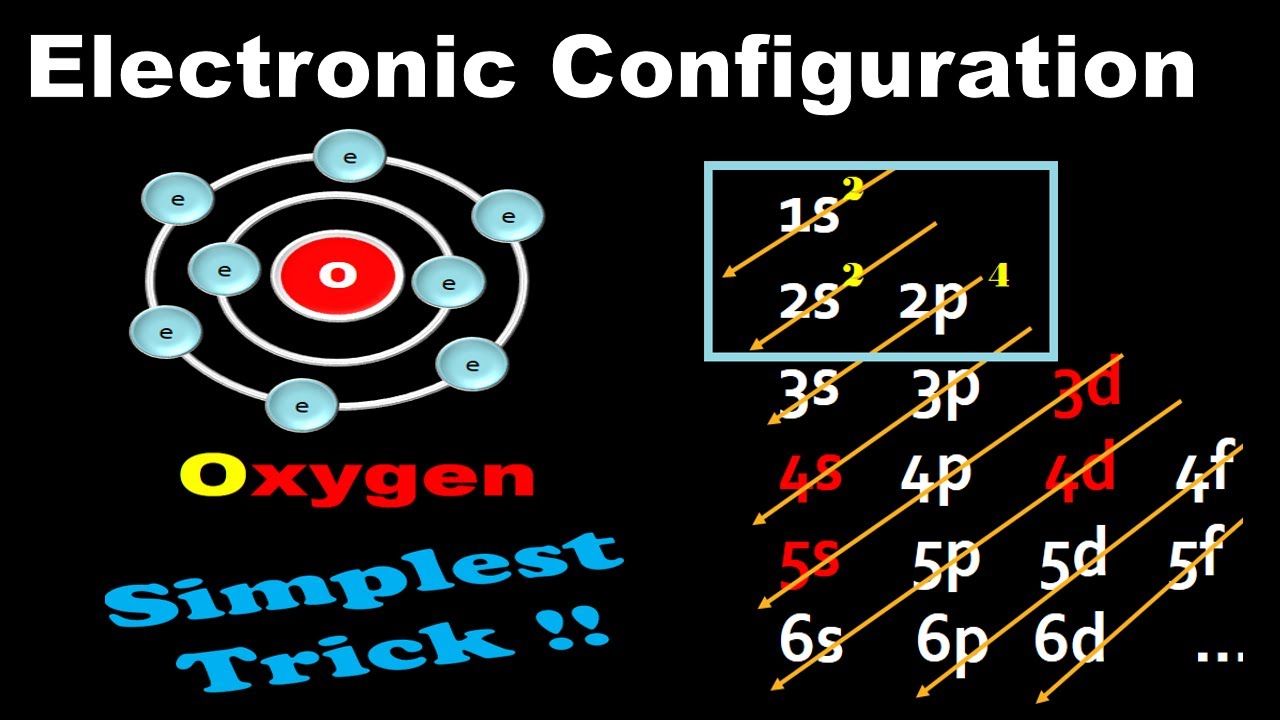
Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Electronic configuration of Oxygen: The electronic configuration of Oxygen is 1 s 2 2 s 2 2 p 4. Oxygen requires two electrons to attain noble gas configuration. Suggest Corrections 24

Electron Configuration for Oxygen (O, O2 ion)
The electron configuration of an oxygen atom [He] 2s 2 2p 4 suggests that neutral oxygen atoms can achieve an octet of valence electrons by sharing two pairs of electrons to form an O=O double bond, as shown in the figure below. According to this Lewis structure, all of the electrons in the O 2 molecule are paired.

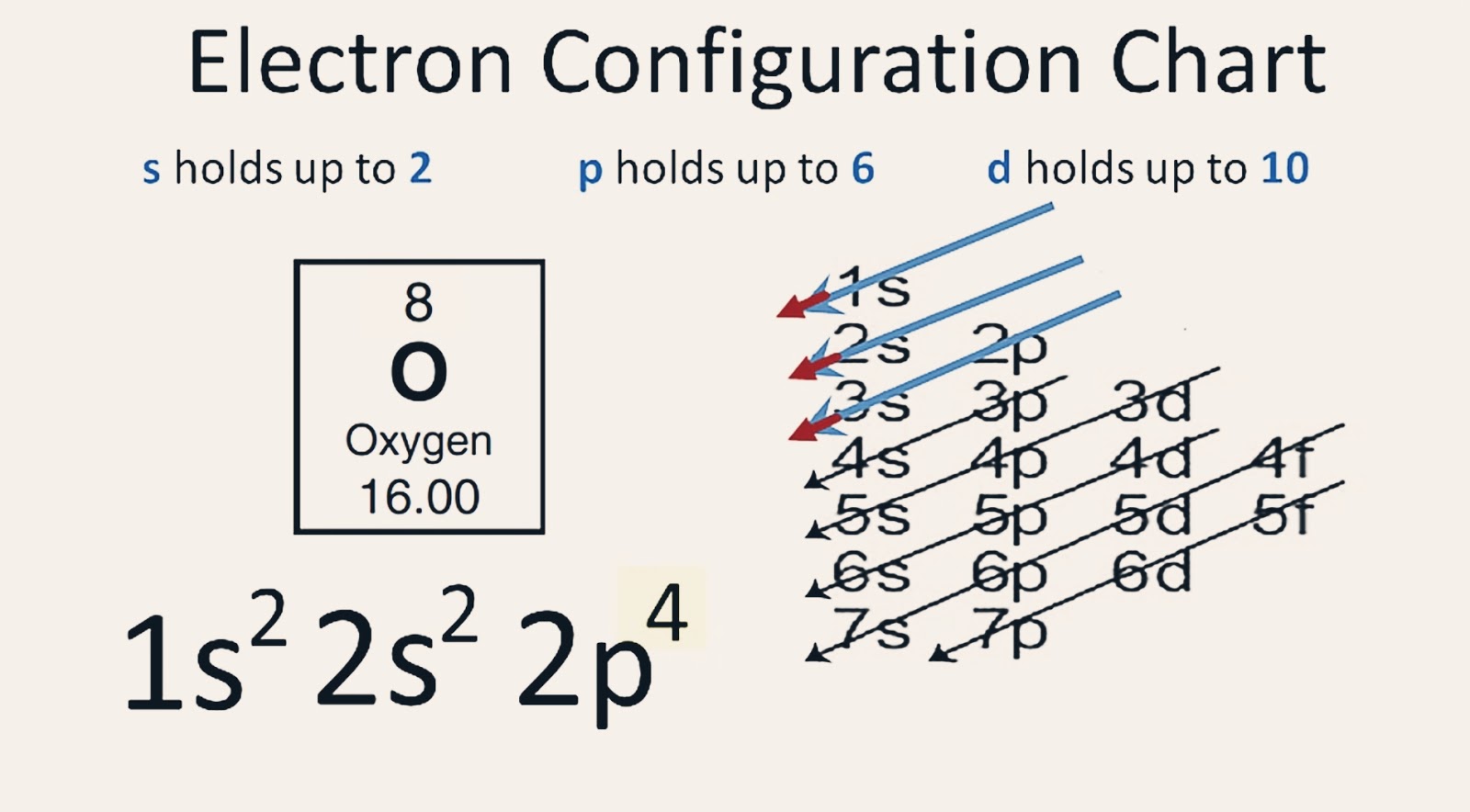
The electron configuration of oxygen is 1s2,2s2 2p4. Science
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,
Oxygen(O) electron configuration and orbital diagram (2022)
What is the electron configuration for oxygen? Chemistry Electron Configuration Electron Configuration 1 Answer Vishwanath Taykhande Oct 10, 2014 The electronic configuration of oxygen is- 1s22s22p4 Note:- For writing the electronic configuration of elements, the Aufbau Principle is used.
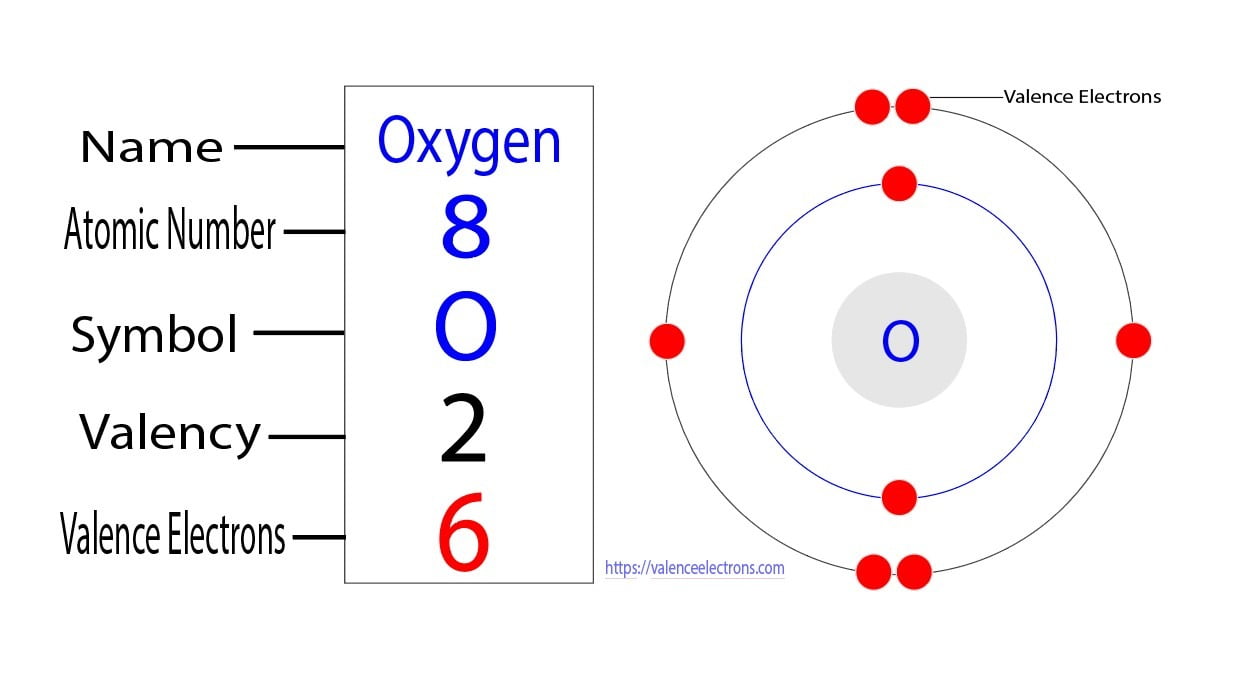
How to Find the Valence Electrons for Oxygen (O)?
Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from solving the Schrödinger's equation for Bohr's hydrogen.

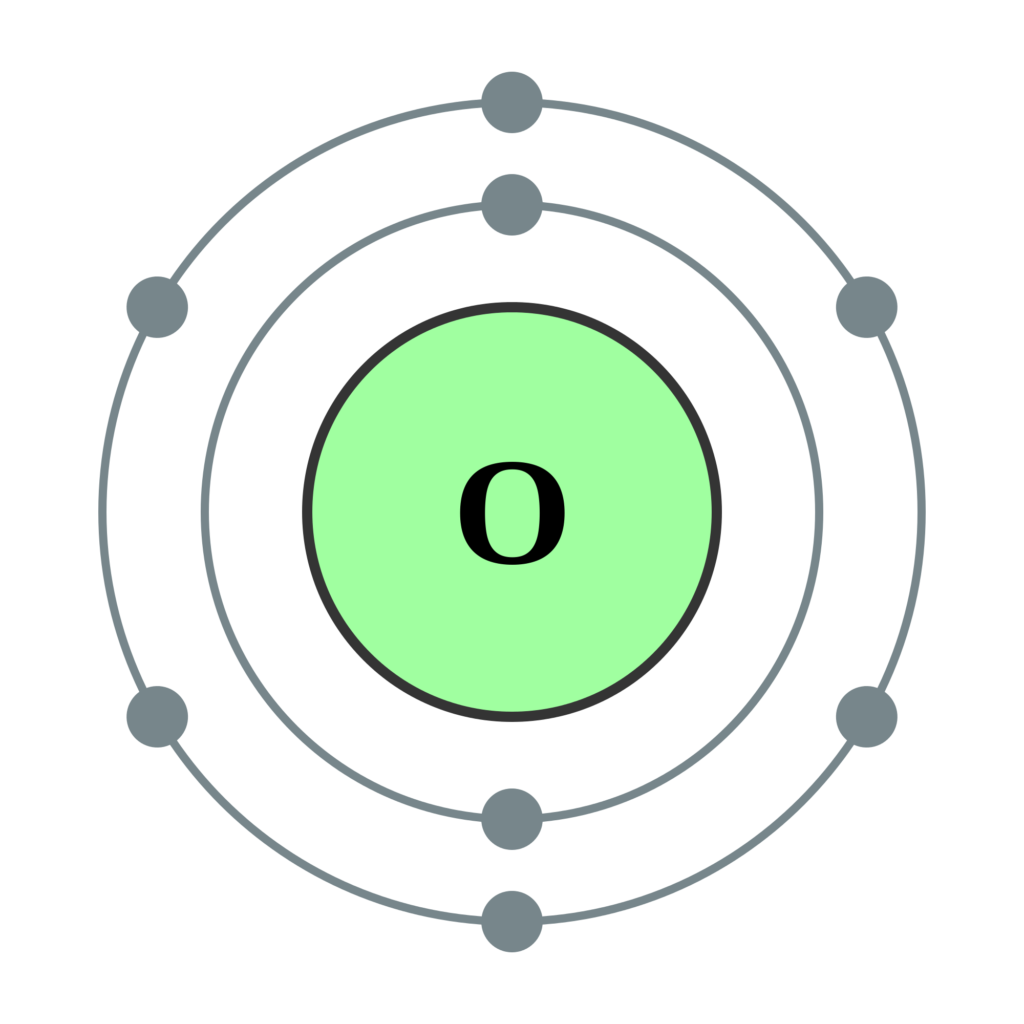
Oxygen Bohr Model (Diagram, Steps To Draw) Techiescientist
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.

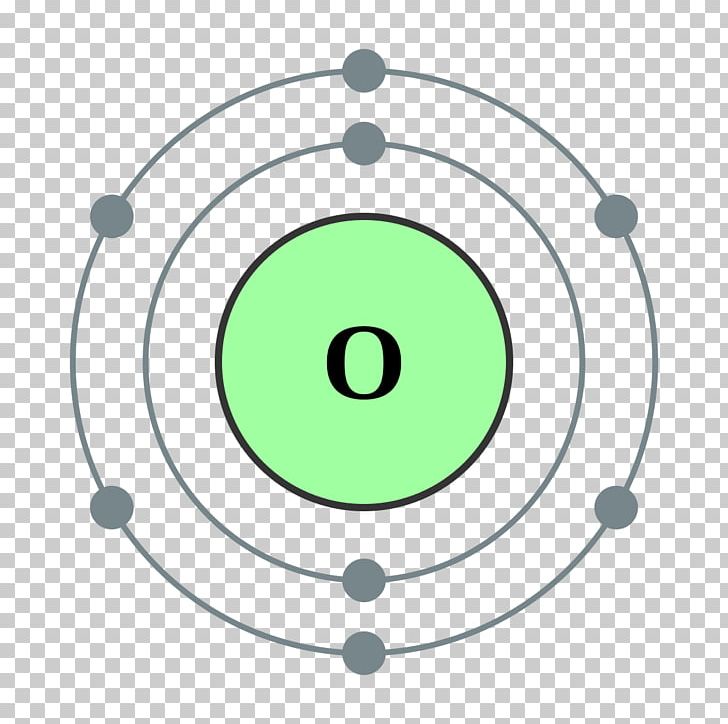
Symbol and electron diagram for Oxygen Royalty Free Vector
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period table or an electron configuration chart. How to Write the Electron Configuration for Oxygen Oxygen is the eighth element with a total of 8 electrons.

Electronic configuration of the oxygen atom Download Scientific Diagram
Example 1.6.3 1.6. 3: Carbon and Oxygen. Consider the electron configuration for carbon atoms: 1s 2 2s 2 2p 2: The two 2s electrons will occupy the same orbital, whereas the two 2p electrons will be in different orbital (and aligned the same direction) in accordance with Hund's rule. Consider also the electron configuration of oxygen.
Bohr Model Chemical Element Oxygen Atomic Theory PNG, Clipart, Angle
If we look at the element after nitrogen in the same period, oxygen (Z = 8) its electron configuration is: 1s 2 2s 2 2p 4 (for an atom). Oxygen has one more electron than nitrogen and as the orbitals are all half filled the electron must pair up. Occupation of Orbitals.
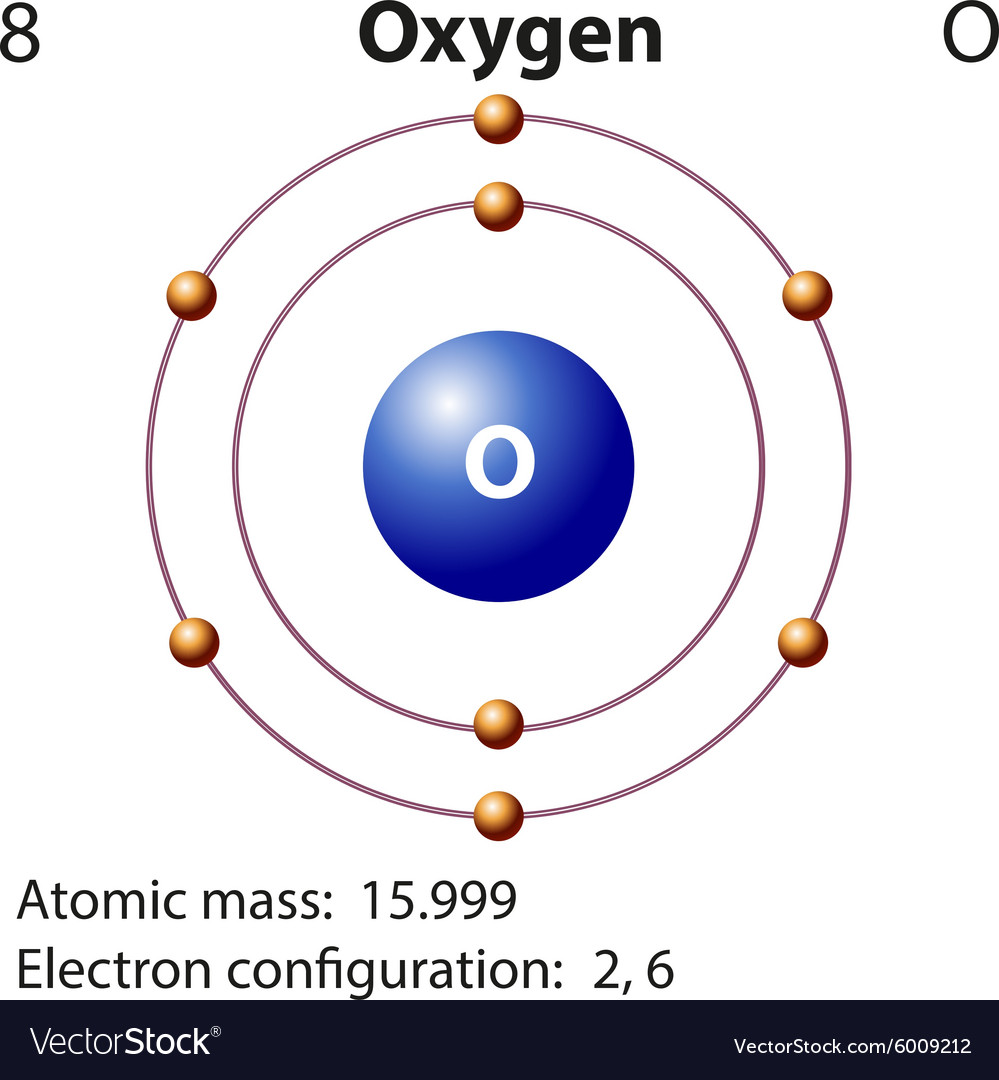
Diagram representation element oxygen Royalty Free Vector
The arrangement of electrons in oxygen in specific rules in different orbits and orbitals is called the electron configuration of oxygen. The electron configuration of oxygen is [ He] 2s 2 2p 4, if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle)

Oxygen Atom Science Notes and Projects
Based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. So Oxygen's electron configuration would be O 1s 2 2s 2 2p 4. Special Cases. Configurations of ions present a special case of electron configuration and also demonstrate the reason for the formation of those ions in the first place.
